
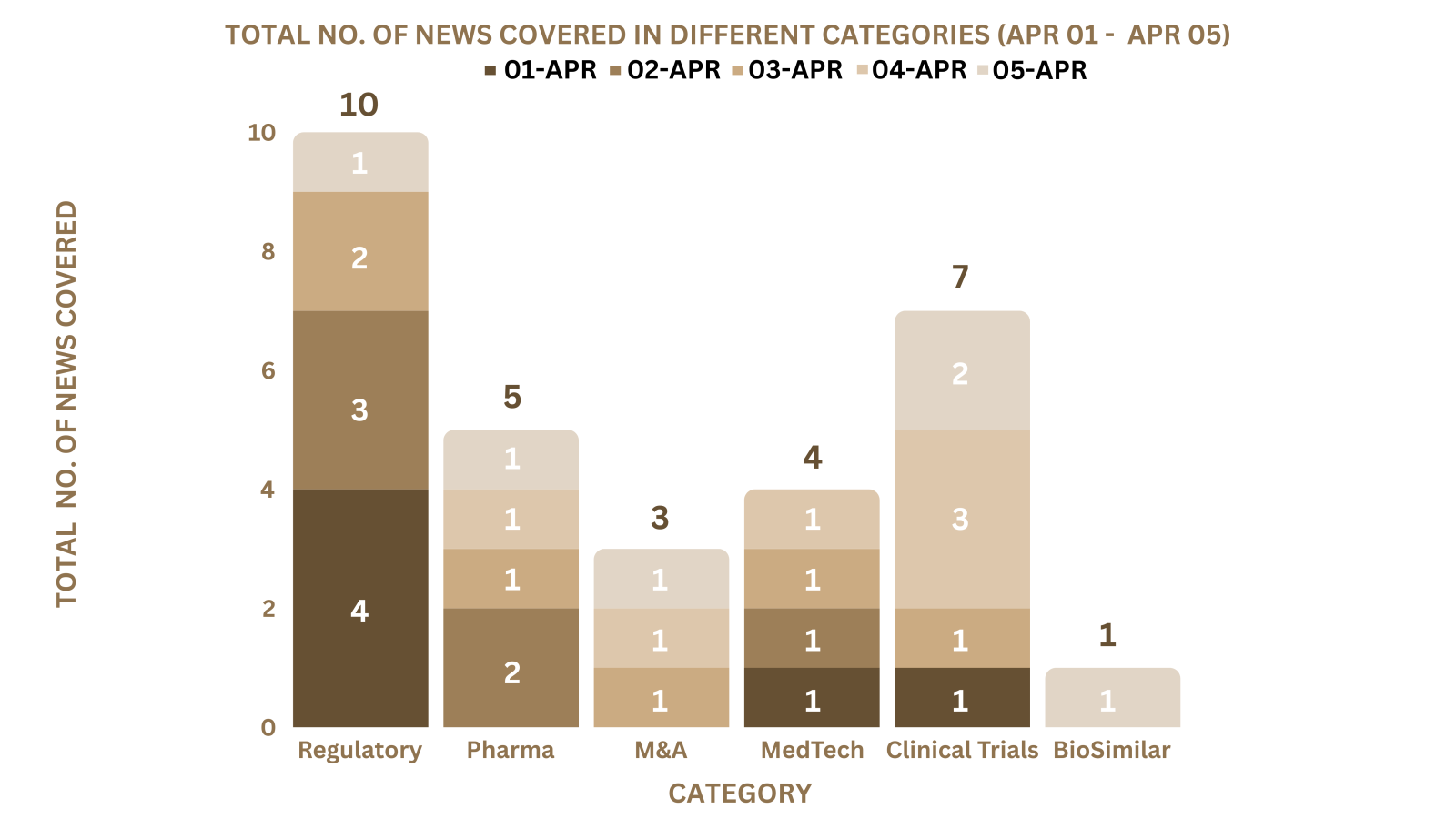
PharmaShots Weekly Snapshots (April 01 – April 05, 2024)
This week PharmaShots’ news was all about the updates on Regulatory, Clinical Trials,Pharma, Pharma, Animal Health & MedTech. Check out our full report below:

The US FDA Approves Gilead’s Vemlidy 25mg Tablets for the Treatment of Chronic Hepatitis B Virus (HBV) Infection in Pediatric Patients
Read More: Gilead
The US FDA Approves AstraZeneca’s Voydeya as an Add-on Therapy to Treat Extravascular Haemolysis in PNH Patients
Read More: AstraZeneca
The US FDA Grants ODD to Biostar Pharma’s Utidelone Injectable to Treat Breast Cancer Brain Metastasis
Read More: Biostar Pharma
Fractyl Health’s IDE for Revita Receives the US FDA Approval for Conducting Remain-1 Study to Treat Obesity
Read More: Fractyl Health
HUTCHMED and Innovent Report the NMPA’s NDA Acceptance for Fruquintinib Plus Sintilimab with Priority Review to Treat Endometrial Cancer
Read More: HUTCHMED
The US FDA Accepts AstraZeneca and Daiichi Sankyo’s BLA of Datopotamab Deruxtecan (Dato-DXd) to Treat Breast Cancer
Read More: AstraZeneca and Daiichi Sankyo’s
The EMA Accepts Rocket Pharmaceuticals’ MAA of RP-L102 for Treating Fanconi Anemia
Read More: Rocket Pharmaceuticals
The EC Expands Approval of BMS’ Reblozyl as a 1L Treatment of Transfusion-Dependent Anemia Due to Myelodysplastic Syndromes
Read More: BMS
Vanda Pharmaceuticals’ Fanapt Gains the US FDA’s Approval to Treat Bipolar I Disorder
Read More: Vanda Pharmaceuticals
The US FDA Accepts UCB’s sBLA of Bimzelx (bimekizumab-bkzx) for the Treatment of Hidradenitis Suppurativa
Read More: UCB

Incyte and China Medical System Collaborate on Povorcitinib for Autoimmune and Inflammatory Dermatologic Indications
Read More: Incyte and China Medical System
Ipsen and Sutro Biopharma Join Forces on STRO-003 for Treating Solid Tumors
Read More: Ipsen and Sutro Biopharma
MaxCyte Partners with Be Biopharma to Develop Engineered B Cell Medicines (BCMs)
Read More: MaxCyte
Caris Life Sciences and Merck KGaA Join Forces to Discover and Develop ADCs for Cancer Treatment
Read More: Caris Life sciences and Merck
MiNA Therapeutics and Nippon Shinyaku Partner on RNAa Therapeutics for Rare Neurodegenerative Diseases
Read More: MiNA Therapeutics and Nippon Shinyaku
For an Aggregate of $1.8B, Genmab to Acquire ProfoundBio for Enhancing its Oncology Portfolio
Read More: Genmab
ARCA Biopharma Merges with Oruka Therapeutics to Develop Biologics for Chronic Skin Diseases
Read More: ARCA & Oruka
For an Aggregate of ~$13.1B, Johnson & Johnson to Acquire Shockwave Medical
Read More: Johnson & Johnson & Shockwave Medical

PassPort Technologies Reveals P-I Study Results of Zolmitriptan Transdermal Microporation System to Treat Acute Migraine
Read More: PassPort Technologies
The US FDA Clears Abbott’s Whole Blood Rapid Test for Evaluating Patients with Suspected Concussion
Read More: Abbott
The US FDA Approves Abbott’s Triclip Transcatheter Edge-To-Edge Repair System to Treat Tricuspid Regurgitation
Read More: Abbott
The US FDA Grants 510(k) Clearance to AngioDynamics’ AlphaVac F18⁸⁵ System for the Treatment of Pulmonary Embolism
Read More:Angiodynamics
BMS Reveals the P-III (YELLOWSTONE) Trial Results of Zeposia for Treating Moderate to Severe Active Crohn’s Disease
Read More: BMS
EnteroBiotix Reports the Initiation of P-II Study Evaluating EBX-102-02 to Treat Irritable Bowel Syndrome (IBS)
Read More: EnteroBiotix
Merck and Daiichi Sankyo Dose First Patient with Raludotatug Deruxtecan in P-II/III (REJOICE-Ovarian01) Study to Treat Ovarian Cancer
Read More: Merck & Daiichi Sankyo
SpliSense Receives the US FDA’s Clearance for SPL84 P-II Clinical Evaluation to Treat Cystic Fibrosis
Read More: SpliSense
Neurocrine Biosciences Reports First Patient Dosing with NBI-1070770 Under the P-II Study to Treat Major Depressive Disorder
Read More: Neurocrine Bioscience
Merck Reports the Commencement of MK-1084’s P-III Study in Combination with Keytruda for Treating Metastatic Non-Small Cell Lung Cancer
Read More: Merck
AstraZeneca Reports the P-III Study Results of Imfinzi for the Treatment of Small Cell Lung Cancer
Read More: AstraZeneca

Teva Pharmaceuticals Partners with mAbxience to Develop Biosimilar Candidates for Treating Oncology Indications
Read More: Teva Pharmaceuticals
Related Post:- PharmaShots Weekly Snapshots (March 26 – March 29, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














